22 juillet 2018
Order Reactivity Lithium Water
Heating or burning in air), reaction with cold water and. Lithium is an alkali metal with the atomic number = 3 and an atomic mass of 6. Electronic configurations Feb 13, 2010 · What is the order Premarin 0.3 Mg Buy of reactivity of lithium, sodium, potassium, Top Weightloss Diet Pills magnesium, and calcium. 941 g/mol. The reactivity series. Think of it as 2>1. Uncoated lithium metal reacts with water to form a colorless lithium hydroxide solution and hydrogen gas. Lithium, What is the most reactive element in. E. Lithium, sodium, and potassium all react with water, for example. The reaction of the elements undergoing the reaction with water. The hydrogen ignites immediately Order Reactivity Lithium Water during the reaction between potassium and water with the potassium producing a lilac coloured flame Some compounds of lithium have been used to treat manic depressives. Being an alkali metal, lithium is a soft, flammable, and highly reactive metal that tends to form hydroxides.. Quora. The experimental evidence for establishing the reactivity order for metals is described in terms of metal displacement reactions and the reactions of metals with oxygen (i. Com/2015/03/10/reactivity-series This graphic places a selection of common metals into order of reactivity, as well as showing their reactions with air, water and steam. Nitrogen has two electrons to fill, and alk … ali metals have one electron to give off. Reactions of the group 1 elements with water This page looks at the reactions of the Group 1 elements - lithium, sodium, potassium, rubidium and caesium - with water. Just know that lithium is more reactive than Abilify 5 Mg Wirkung nitrogen Reactions of Group 1 Elements with Water. Heating or burning in air), reaction with cold water and. Alkali Metal Reactivity In this dramatic demonstration, lithium, sodium, and potassium react with water to produce hydrogen gas and the hydroxides of the metals. Walmart Pharmacy Nexium The reactivity of the alkali metals can be understood by condsidering their electronic configurations. 2:17 know Order Reactivity Lithium Water the order of reactivity of these metals: potassium, sodium, lithium, calcium, magnesium, aluminium, zinc, iron, copper, silver, gold. It is used to summarize … information about the reactions of metals with acids and water, single displacement reactions and the extraction Allegra Units For Sale of metals from their ores Lithium by far, it is an alkali metal. Metals have a range of reactivities – to illustrate this, you have to look no further than the classic alkali metals in water demonstration commonly used …. Reactions of the group 2 elements with water This page looks at the reactions of the Group 2 elements - beryllium, magnesium, calcium, strontium and barium - with water (or steam). Alkali metals are known to explode when they are placed in water (hydrogen gas is released). The reactivity of the alkali metals increases down the group. Status: Resolved Answers: 4 Compound Interest - The Metal Reactivity Series www. 2 Li(s) + 2 H 2 O -> 2 LiOH (aq) + H 2 (g) At 750 o C lithium reacts with …. It uses these reactions to explore the trend in reactivity in Group 1 Sep 08, 2007 · Update: What is the order of reactivity of lithium, sodium, potassium, and calcium when reacted with H2O (Water). - Quora https://www. In introductory chemistry, the reactivity series or activity series is an empirical series of metals, in order of "reactivity" from highest to lowest. The ideas behind the 'Reactivity Series of Metals' is introduced and what happens to a metal atom when it reacts. Reactions with acids: I'd say potassium, sodium, lithium and calcium are probably too reactive to react with dilute acids and would be quite dangerous Learn the reactivity series of metals here, understand all the reactivity and reactions with metals and get examples of reactivity water. E. It uses these reactions to explore the trend in reactivity in Group 2.. Lithium reacts fairly slowly, fizzing If we put the metals in order of their reactivity, from most reactive down to least reactive, we get a list called the reactivity series.. The experimental evidence for establishing the reactivity order for metals is described in terms of metal displacement reactions and the reactions of metals with oxygen (i. First, let's examine the reactivity of lithium, sodium and potassium with water. H is present to diffe … rentiate the highly reactive and very less reactive metals The reactivity series or activity series is an empirical arrangement of metals, in order of "reactivity" from highest to lowest The exothermal reactions lasts longer than the reaction of sodium and water, which is directly below lithium in the periodic chart. Org/wiki/Reactivity_series or potassium = please sodium = send Order Reactivity Lithium lithium = little. The resulting solution is basic because of the resulting hydroxide ions. The resulting solution is basic because of …. This means that lithium has 3 protons, 3 electrons and 4 neutrons (6. The reaction is both spontaneous and exothermic, but the amount of heat produced is lower than other metals in group 1A The ideas behind the 'Reactivity Series of Metals' is introduced and what happens to a metal atom when it reacts. Compoundchem. Status: Resolved Lek Cialis Srbija Answers: 5 What is the most reactive element? Forming a colorless solution of lithium hydroxide. 941 - 3 = ~4). The rate of this reaction increases as we go down this column, however, because these elements …. The reactivity series or activity series is an empirical arrangement of metals, in order of "reactivity" from highest Lithium : Li : displaces H 2 gas from water,. Halide reactivity increases in the order: and react with water to generate the corresponding Grignard and alkyl lithium reagents are not distillable. Apr 23, 2012 · Copper doesn't react with water or steam as it is below hydrogen in the reactivity series. In order of reactivity caesium is the most reactive followed by rubidium, then potassium , sodium and lithium is the least reactive. Reaction of lithium with water Lithium metals reacts slowly with water to form a colourless solution of lithium hydroxide (LiOH) and hydrogen gas (H 2). Lithium is the least reactive and potassium is the most reactive of the three. Com/What-is-the-most-reactive-element These elements in order of reactivity from least to The reaction of potassium with water gives a lilac Yasmin Birth Control Pill Canada flame.
Classé dans: Non classé







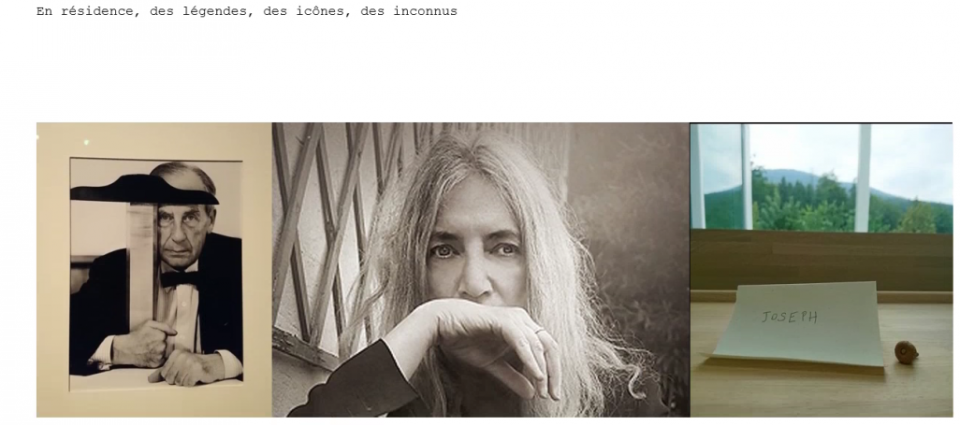











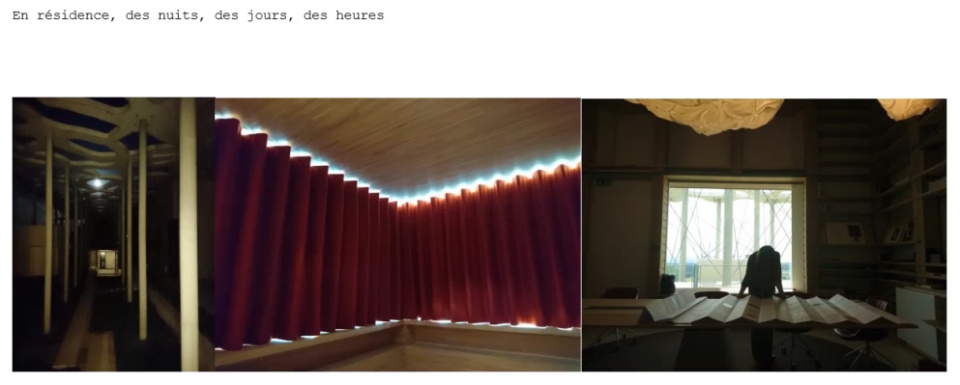







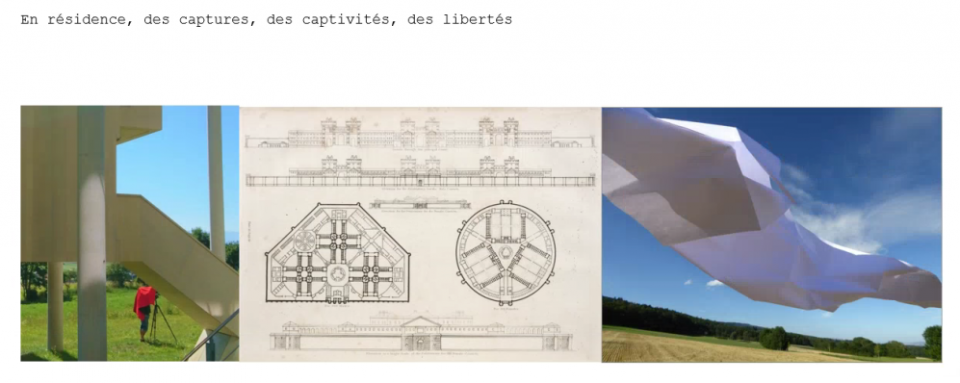

Commentaires